Motor Gasoline Definition:
A complex mixture of relatively volatile hydrocarbons with or without small quantities of additives, blended to form a fuel suitable for use in spark-ignition engines. Motor gasoline, as defined in ASTM Specification D 4814 or Federal Specification VV-G-1690C, is characterized as having a boiling range of 122 to 158 degrees Fahrenheit at the 10 percent recovery point to 365 to 374 degrees Fahrenheit at the 90 percent recovery point.
Motor Gasoline includes conventional gasoline; all types of oxygenated gasoline, including gasohol; and reformulated gasoline, but excludes aviation gasoline.
Note: Volumetric data on blending components, such as oxygenates, are not counted in data on finished motor gasoline until the blending components are blended into the gasoline.
What is Petroleum?
Petroleum (L. petroleum, from Greek: πέτρα (rock) + Latin: oleum (oil) is a naturally occurring, yellow-to-black liquid found in geologic formations beneath the Earth's surface, which is commonly refined into various types of fuels. It consists of hydrocarbons of various molecular weights and other liquid organic compounds.The name petroleum covers both naturally occurring unprocessed crude oil and petroleum products that are made up of refined crude oil. A fossil fuel, petroleum is formed when large quantities of dead organisms, usually zooplankton and algae, are buried underneath sedimentary rock and subjected to intense heat and pressure.
Petroleum is recovered mostly through oil drilling. This comes after the studies of structural geology (at the reservoir scale), sedimentary basin analysis, reservoir characterization (mainly in terms of the porosity and permeability of geologic reservoir structures). It is refined and separated, most easily by boiling point, into a large number of consumer products, from gasoline (petrol) and kerosene to asphalt and chemical reagents used to make plastics and pharmaceuticals. Petroleum is used in manufacturing a wide variety of materials, and it is estimated that the world consumes about 90 million barrels each day.
The use of fossil fuels such as petroleum has a negative impact on Earth's biosphere, releasing pollutants and greenhouse gases into the air and damaging ecosystems through events such as oil spills. Concern over the depletion of the earth's finite reserves of oil, and the effect this would have on a society dependent on it, is a concept known as peak oil.
Composition
In its strictest sense, petroleum includes only crude oil, but in common usage it includes all liquid, gaseous, and solid hydrocarbons. Under surface pressure and temperature conditions, lighter hydrocarbons methane, ethane, propane and butane occur as gases, while pentane and heavier ones are in the form of liquids or solids. However, in an underground oil reservoir the proportions of gas, liquid, and solid depend on subsurface conditions and on the phase diagram of the petroleum mixture.
An oil well produces predominantly crude oil, with some natural gas dissolved in it. Because the pressure is lower at the surface than underground, some of the gas will come out of solution and be recovered (or burned) as associated gas or solution gas. A gas well produces predominantly natural gas. However, because the underground temperature and pressure are higher than at the surface, the gas may contain heavier hydrocarbons such as pentane, hexane, and heptane in the gaseous state. At surface conditions these will condense out of the gas to form natural gas condensate, often shortened to condensate. Condensate resembles petrol in appearance and is similar in composition to some volatile light crude oils.
The proportion of light hydrocarbons in the petroleum mixture varies greatly among different oil fields, ranging from as much as 97 percent by weight in the lighter oils to as little as 50 percent in the heavier oils and bitumens.
The hydrocarbons in crude oil are mostly alkanes, cycloalkanes and various aromatic hydrocarbons while the other organic compounds contain nitrogen, oxygen and sulfur, and trace amounts of metals such as iron, nickel, copper and vanadium. The exact molecular composition varies widely from formation to formation but the proportion of chemical elements vary over fairly narrow limits as follows.
| Element | Percent range |
|---|---|
| Carbon | 83 to 85% |
| Hydrogen | 10 to 14% |
| Nitrogen | 0.1 to 2% |
| Oxygen | 0.05 to 1.5% |
| Sulfur | 0.05 to 6.0% |
| Metals | < 0.1% |
Four different types of hydrocarbon molecules appear in crude oil. The relative percentage of each varies from oil to oil, determining the properties of each oil.
| Hydrocarbon | Average | Range |
|---|---|---|
| Alkanes (paraffins) | 30% | 15 to 60% |
| Naphthenes | 49% | 30 to 60% |
| Aromatics | 15% | 3 to 30% |
| Asphaltics | 6% | remainder |
Crude oil varies greatly in appearance depending on its composition. It is usually black or dark brown (although it may be yellowish, reddish, or even greenish). In the reservoir it is usually found in association with natural gas, which being lighter forms a gas cap over the petroleum, and saline water which, being heavier than most forms of crude oil, generally sinks beneath it. Crude oil may also be found in semi-solid form mixed with sand and water, as in the Athabasca oil sands in Canada, where it is usually referred to as crude bitumen. In Canada, bitumen is considered a sticky, black, tar-like form of crude oil which is so thick and heavy that it must be heated or diluted before it will flow. Venezuela also has large amounts of oil in the Orinoco oil sands, although the hydrocarbons trapped in them are more fluid than in Canada and are usually called extra heavy oil. These oil sands resources are called unconventional oil to distinguish them from oil which can be extracted using traditional oil well methods. Between them, Canada and Venezuela contain an estimated 3.6 trillion barrels (570×109 m3) of bitumen and extra-heavy oil, about twice the volume of the world's reserves of conventional oil.
Petroleum is used mostly, by volume, for producing fuel oil and petrol, both important "primary energy" sources. 84 percent by volume of the hydrocarbons present in petroleum is converted into energy-rich fuels (petroleum-based fuels), including petrol, diesel, jet, heating, and other fuel oils, and liquefied petroleum gas.The lighter grades of crude oil produce the best yields of these products, but as the world's reserves of light and medium oil are depleted, oil refineries are increasingly having to process heavy oil and bitumen, and use more complex and expensive methods to produce the products required. Because heavier crude oils have too much carbon and not enough hydrogen, these processes generally involve removing carbon from or adding hydrogen to the molecules, and using fluid catalytic cracking to convert the longer, more complex molecules in the oil to the shorter, simpler ones in the fuels.
Due to its high energy density, easy transportability and relative abundance, oil has become the world's most important source of energy since the mid-1950s. Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics; the 16 percent not used for energy production is converted into these other materials. Petroleum is found in porous rock formations in the upper strata of some areas of the Earth's crust. There is also petroleum in oil sands (tar sands). Known oil reserves are typically estimated at around 190 km3 (1.2 trillion (short scale) barrels) without oil sands, or 595 km3 (3.74 trillion barrels) with oil sands. Consumption is currently around 84 million barrels (13.4×106 m3) per day, or 4.9 km3 per year. Which in turn yields a remaining oil supply of only about 120 years , if current demand remain static.
Petroleum is a mixture of a very large number of different hydrocarbons; the most commonly found molecules are alkanes (paraffins),cycloalkanes (naphthenes), aromatic hydrocarbons, or more complicated chemicals like asphaltenes. Each petroleum variety has a unique mix of molecules, which define its physical and chemical properties, like color and viscosity.
The alkanes, also known as paraffins, are saturated hydrocarbons with straight or branched chains which contain only carbon andhydrogen and have the general formula CnH2n+2. They generally have from 5 to 40 carbon atoms per molecule, although trace amounts of shorter or longer molecules may be present in the mixture.
The alkanes, also known as paraffins, are saturated hydrocarbons with straight or branched chains which contain only carbon andhydrogen and have the general formula CnH2n+2. They generally have from 5 to 40 carbon atoms per molecule, although trace amounts of shorter or longer molecules may be present in the mixture.
The alkanes from pentane (C5H12) to octane (C8H18) are refined into petrol, the ones from nonane (C9H20) to hexadecane (C16H34) intodiesel fuel, kerosene and jet fuel. Alkanes with more than 16 carbon atoms can be refined into fuel oil and lubricating oil. At the heavier end of the range, paraffin wax is an alkane with approximately 25 carbon atoms, while asphalt has 35 and up, although these are usually cracked by modern refineries into more valuable products. The shortest molecules, those with four or fewer carbon atoms, are in a gaseous state at room temperature. They are the petroleum gases. Depending on demand and the cost of recovery, these gases are either flared off, sold as liquified petroleum gas under pressure, or used to power the refinery's own burners. During the winter, butane (C4H10), is blended into the petrol pool at high rates, because its high vapor pressure assists with cold starts. Liquified under pressure slightly above atmospheric, it is best known for powering cigarette lighters, but it is also a main fuel source for many developing countries. Propane can be liquified under modest pressure, and is consumed for just about every application relying on petroleum for energy, from cooking to heating to transportation.
The cycloalkanes, also known as naphthenes, are saturated hydrocarbons which have one or more carbon rings to which hydrogen atoms are attached according to the formula CnH2n. Cycloalkanes have similar properties to alkanes but have higher boiling points.
The aromatic hydrocarbons are unsaturated hydrocarbons which have one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are attached with the formula CnHn. They tend to burn with a sooty flame, and many have a sweet aroma. Some are carcinogenic.
These different molecules are separated by fractional distillation at an oil refinery to produce petrol, jet fuel, kerosene, and other hydrocarbons. For example, 2,2,4-trimethylpentane(isooctane), widely used in petrol, has a chemical formula of C8H18 and it reacts with oxygen exothermically:
- 2 C
8H
18(l) + 25 O
2(g) → 16 CO
2(g) + 18 H
2O(g) (ΔH = −5.51 MJ/mol of octane)
The number of various molecules in an oil sample can be determined in laboratory. The molecules are typically extracted in a solvent, then separated in a gas chromatograph, and finally determined with a suitable detector, such as a flame ionization detector or a mass spectrometer. Due to the large number of co-eluted hydrocarbons within oil, many cannot be resolved by traditional gas chromatography and typically appear as a hump in the chromatogram. This unresolved complex mixture (UCM) of hydrocarbons is particularly apparent when analysing weathered oils and extracts from tissues of organisms exposed to oil.
Incomplete combustion of petroleum or petrol results in production of toxic byproducts. Too little oxygen results in carbon monoxide. Due to the high temperatures and high pressures involved, exhaust gases from petrol combustion in car engines usually include nitrogen oxides which are responsible for creation of photochemical smog.
Rate of world energy usage per day, from 1970 to 2010. 1000TWh=1PWh
Global fossil carbon emissions, an indicator of consumption, for 1800–2007. Total is black, Oil is in blue.
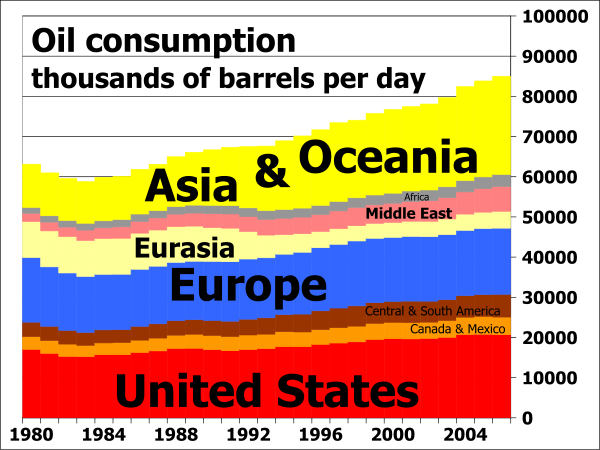
Daily oil consumption from 1980 to 2006
Oil consumption per day by region
Oil consumption by percentage of total per region from 1980 to 2006: red=USA, blue=Europe, yellow=Asia+Oceania




No comments:
Post a Comment